What is the most important information I should know about PALYNZIQ?
PALYNZIQ can cause a severe allergic reaction (anaphylaxis) that may be life–threatening and may happen any time during treatment. Severe allergic reactions are a serious and common side effect of PALYNZIQ.
You will get your first injection under the supervision of a healthcare provider prepared to manage a severe allergic reaction. You will be watched for at least 1 hour after your injection for a severe allergic reaction.
- If you have a severe allergic reaction during treatment with PALYNZIQ, you will need to receive an immediate administration of epinephrine and get emergency medical help right away.
- Your healthcare provider will decide if you (or your caregiver) are able to give PALYNZIQ injections, to recognize the signs and symptoms of a severe allergic reaction, and how to use your epinephrine and call for medical help, if needed.
- Your healthcare provider may ask an adult observer (or your caregiver) to be with you during and for at least 1 hour after each injection to watch for signs of a severe allergic reaction. If needed, they should be ready to give you epinephrine and call 911 to get medical help right away.
Stop PALYNZIQ and get emergency medical care right away if you have any of the following signs or symptoms of a severe allergic reaction during treatment with PALYNZIQ: fainting (passing out); dizziness or lightheadedness; sudden confusion; trouble breathing or wheezing; chest discomfort or chest tightness; fast heart rate; swelling of your face, lips, eyes, or tongue; throat swelling or tightness; flushed or red skin; skin rash, itching, or raised bumps on skin; nausea, vomiting, or diarrhea; losing control of urine or stools.
- Your healthcare provider will give you a prescription for epinephrine and teach you (or your caregiver) and your observer, if needed, how to use it if you have a severe allergic reaction. Keep epinephrine with you at all times during treatment with PALYNZIQ. Read the Patient Information that comes with epinephrine for more details.
- If you have a severe allergic reaction, stop taking PALYNZIQ until you talk with your healthcare provider. Tell them that you had a severe allergic reaction. They will decide if it is safe for you to keep using it.
- Your healthcare provider may prescribe other medicines to take before your PALYNZIQ injection to help reduce the symptoms of an allergic reaction.
- If your healthcare provider decides you can keep taking PALYNZIQ, you will receive your next injection under the supervision of a healthcare provider prepared to manage a severe allergic reaction, where you will be closely watched for at least 1 hour after your injection for signs of a severe allergic reaction.
- Your healthcare provider will give you a PALYNZIQ Patient Wallet Card that lists the signs and symptoms of a severe allergic reaction. You (or your caregiver), or your observer, should call 911 right away if any of these signs or symptoms occur. Keep this card with you at all times while using PALYNZIQ. Show this card to any other healthcare provider who treats you.
PALYNZIQ REMS: PALYNZIQ is only available through a restricted program called the PALYNZIQ REMS (Risk Evaluation and Mitigation Strategy). Before you can receive PALYNZIQ, you must:
- enroll in the program.
- learn about the risk of a severe allergic reaction (anaphylaxis) from a healthcare provider certified in the PALYNZIQ REMS so you can understand the risks and benefits of treatment.
- fill a prescription of epinephrine and carry it with you at all times while using PALYNZIQ.
- keep the PALYNZIQ Patient Wallet Card with you at all times.
What should I tell my healthcare provider BEFORE starting PALYNZIQ?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Tell your healthcare provider about all of your medical conditions, including if you:
- cannot or do not want to use epinephrine to treat a severe allergic reaction.
- are pregnant or plan to become pregnant. It is not known whether PALYNZIQ will harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you might be pregnant while taking PALYNZIQ.
- If your Phe levels are too high or too low during pregnancy, this may also affect your unborn baby. You and your healthcare provider can decide the best way for you to manage your blood Phe levels and if taking PALYNZIQ is right for you and your unborn baby.
- It is very important to keep your Phe at the levels your healthcare provider recommends during pregnancy.
- Pregnancy Surveillance Program. There is a pregnancy surveillance program for females who take PALYNZIQ during pregnancy, or who become pregnant while taking PALYNZIQ or within 1 month after their last dose of PALYNZIQ. This program collects information about the health of you and your baby while taking PALYNZIQ. To join, talk to your healthcare provider or call BioMarin at 1-866-906-6100.
- are breastfeeding or plan to breastfeed. It is not known if PALYNZIQ passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take PALYNZIQ.
What are the possible side effects of PALYNZIQ?
PALYNZIQ may cause serious side effects, including:
- Serious allergic reactions. See “What is the most important information I should know about PALYNZIQ?”
- Other allergic reactions to PALYNZIQ can happen during treatment. Call your healthcare provider right away if you have any of the following symptoms of an allergic reaction including: rash, itching, or swelling of the face, lips, eyes, or tongue. Your healthcare provider may change your dose of PALYNZIQ, pause your treatment, or give you medicine for you to take before your injection to help reduce the symptoms of an allergic reaction.
-
Injection site infections. Serious infections at the injection site have happened in people during treatment with PALYNZIQ. Some of these injection site infections required hospitalization, surgery, treatment with antibiotics given through the vein, or stopping treatment with PALYNZIQ. Change (rotate) your injection site and check your injection site for redness, swelling, or tenderness before each injection. Tell your healthcare provider right away if you develop signs or symptoms of an infection at your injection site that are new, do not go away, or get worse, including: pain, redness, swelling, or tenderness; the area feels hard; fluid or pus; blisters; a dark scab; an open wound.
Do not inject PALYNZIQ into the affected area until the infection has cleared.
- Low levels of Phe in your blood (hypophenylalaninemia, or HypoPhe). Your healthcare provider will monitor your blood Phe levels during treatment.
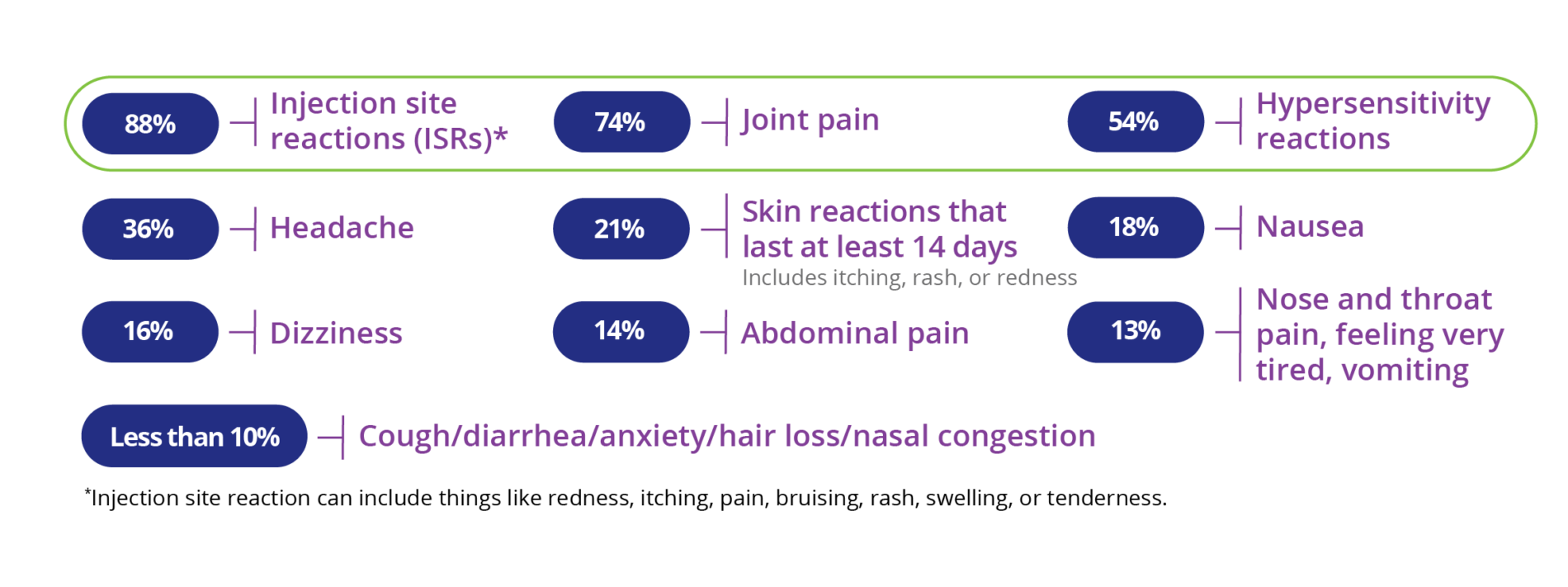
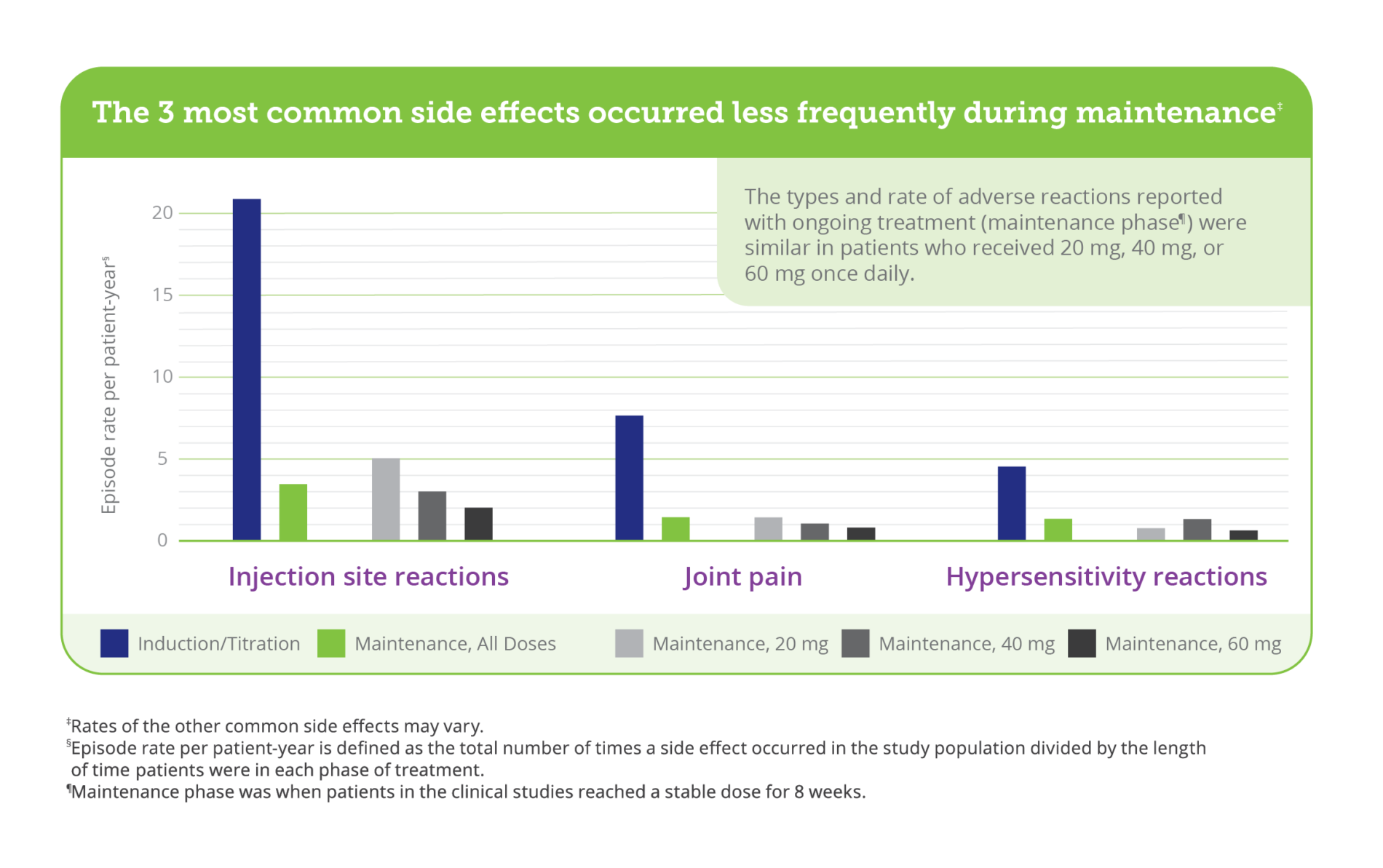
The most common side effects of PALYNZIQ include: injection site reactions: redness, itching, pain, bruising, rash, swelling, tenderness; joint pain; allergic reactions; headache; skin reactions that spread and last at least 14 days, such as itching, rash, redness; nausea; stomach pain; vomiting; cough; mouth and throat pain; itching; diarrhea; stuffy nose; feeling very tired; dizziness; anxiety; low levels of Phe in your blood.
The most common side effects of PALYNZIQ in people 12 years to less than 18 years of age include: injection site reactions: redness, itching, pain, bruising, rash, swelling, tenderness; joint pain; headache; fever; allergic reactions; dizziness; nausea; vomiting; feeling very tired; pain in your arms or legs.
These are not all the possible side effects of PALYNZIQ. Call your healthcare provider for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Important notes
Blood Phe testing and diet
- Your healthcare provider will monitor your blood Phe levels during PALYNZIQ treatment.
- Monitor the amount of protein and Phe you eat or drink. Your healthcare provider may change your diet based on the amount of Phe in your blood. Follow your healthcare provider’s instructions about the amount of protein and Phe you should have in your diet.
Missed dose
- If a dose is missed, take the next dose at the regular time. Do not take 2 doses of PALYNZIQ to make up for the missed dose.
Please see full Prescribing Information, including Important Warning, and the Medication Guide.
COM-ET-1199 02/26