You’ll start by taking PALYNZIQ once a week. You’ll self-inject PALYNZIQ just under the skin (subcutaneously). Then, your clinic team will adjust your dose based on how you respond to treatment.
PALYNZIQ comes in prefilled syringes and is available in 3 dosage strengths:


The needle used for PALYNZIQ injections is just a half an inch long. It is injected right under the skin (subcutaneously). This can make it less painful than a flu shot, which is injected into your muscle.

Learn about injecting and administering PALYNZIQ with this step-by-step video.
Get more comfortable with needles and injections with a brief overview of the product, education, and support that you’ll receive when taking PALYNZIQ.
Watch adults with PKU share how their PALYNZIQ injection fits into their daily schedules.
Before injecting PALYNZIQ, talk to your healthcare provider right away if you cannot or will not use auto-injectable epinephrine to treat a severe allergic reaction. If you are pregnant or plan to become pregnant while taking PALYNZIQ, talk to your healthcare provider to discuss the risks and benefits of taking PALYNZIQ during pregnancy to you and your unborn baby. If you are breastfeeding or plan to breastfeed, talk to your healthcare provider to discuss the risks and benefits of taking PALYNZIQ while breastfeeding for you and your baby.
*You must carry your auto-injectable epinephrine with you at all times.
Because of the unique way PALYNZIQ works, it can cause a reaction. When a new treatment enters the body, the immune system doesn’t know what it is and may react to it. This reaction is called a “hypersensitivity event.”
A severe allergic reaction (anaphylaxis) is a type of hypersensitivity event that may be life-threatening and can happen at any time during treatment with PALYNZIQ.
Your clinic team will prescribe autoinjectable epinephrine in case you experience a severe allergic reaction.
They will also train you about how to recognize the signs of a severe allergic reaction so you can be prepared to manage it if it happens.
These signs include:
In clinical trials, about 1 in 2 people who experienced a severe allergic reaction used an autoinjectable epinephrine pen to manage it. If you experience any of these symptoms, reach out to your clinic team right away.
Remember: If you do have a serious allergic reaction with PALYNZIQ, it doesn’t always mean it will happen again. It also does not mean you need to stop taking PALYNZIQ.

Change (rotate) your injection site and check your injection site for redness, swelling, or tenderness before each injection. Tell your healthcare provider right away if you develop signs or symptoms of an infection at your injection site that are new, do not go away, or get worse.

This video will help you learn more about the potential risk of anaphylaxis with PALYNZIQ and how your care team will ensure you know how to respond if it happens.
your Phe levels so your clinic team can see how PALYNZIQ is working for you.
all your appointments so your clinic team can adjust your treatment plan.
all your medicines as prescribed. These can help reduce the risk of side effects.
a consistent diet. Don’t make changes unless your clinic team tells you to do so.
the signs of a severe allergic reaction.
your autoinjectable epinephrine with you at all times.
You can get PALYNZIQ only through the PALYNZIQ REMS program. The purpose of the program is to ensure that you have been fully informed about the risk of anaphylaxis associated with taking PALYNZIQ, and that you understand this risk before you start treatment. Your clinic team can tell you more about PALYNZIQ REMS and help you enroll.
PALYNZIQ can cause a severe allergic reaction (anaphylaxis) that may be life threatening and can happen any time during treatment with PALYNZIQ.
Severe allergic reactions are a serious but common side effect of PALYNZIQ.
Stop injecting PALYNZIQ and get emergency medical care right away if you have any of the following symptoms:
Keep the auto-injectable epinephrine with you at all times during treatment with PALYNZIQ. Read the Patient Information that comes with the auto-injectable epinephrine that your healthcare provider prescribes for you for more information.
If you have a severe allergic reaction, do not continue to take PALYNZIQ until you talk with your healthcare provider. Your healthcare provider will tell you if you can continue treatment with PALYNZIQ.
People taking PALYNZIQ have also experienced allergic reactions other than anaphylaxis. Talk to your healthcare provider if you experience any allergic reactions when taking PALYNZIQ.
PALYNZIQ REMS: PALYNZIQ is available only through a restricted program called the PALYNZIQ REMS (Risk Evaluation and Mitigation Strategy). Talk to your healthcare provider about the PALYNZIQ REMS and how to enroll.
What should I tell my healthcare provider BEFORE starting PALYNZIQ?
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before injecting PALYNZIQ, talk to your healthcare provider right away if you cannot or will not use auto-injectable epinephrine to treat a severe allergic reaction. If you are pregnant or plan to become pregnant while taking PALYNZIQ, talk to your healthcare provider to discuss the risks and benefits of taking PALYNZIQ during pregnancy to you and your unborn baby. If you are breastfeeding or plan to breastfeed, talk to your healthcare provider about the best way to feed your baby if you take PALYNZIQ.
Before injecting PALYNZIQ, read the Medication Guide and Instructions for Use that come with your PALYNZIQ injection.
What should I watch for AFTER starting PALYNZIQ?
PALYNZIQ may cause serious side effects, including:
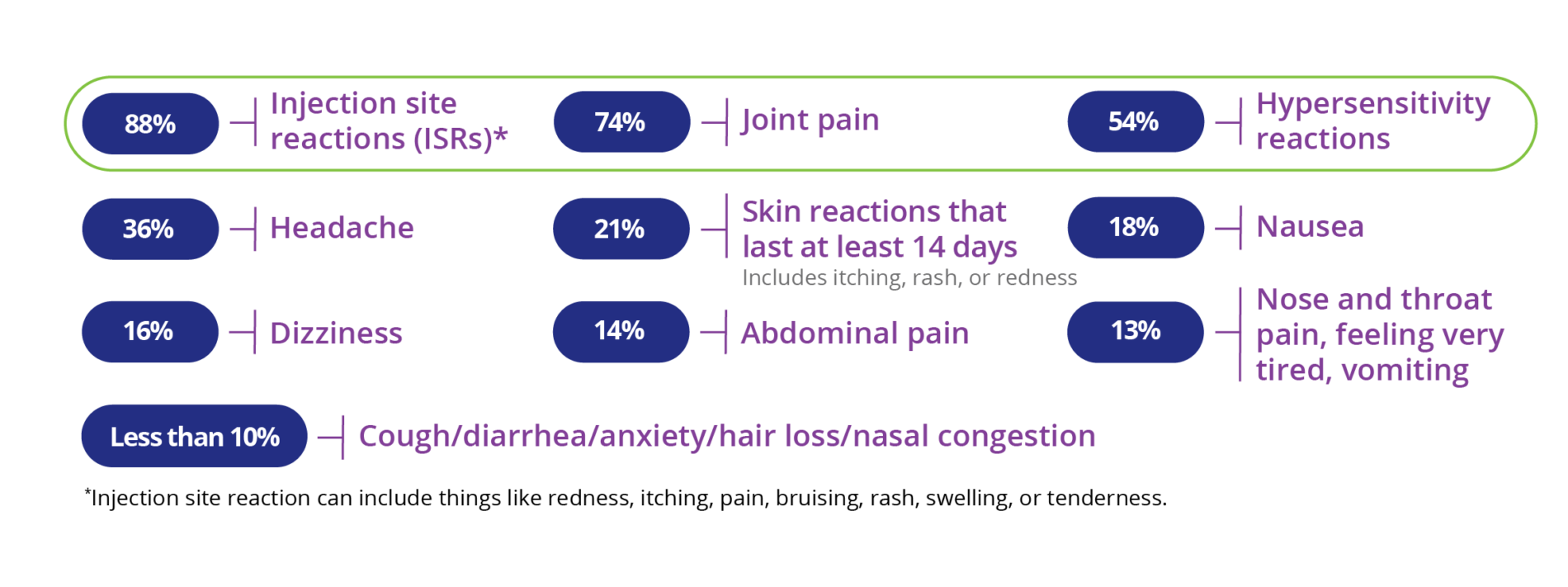
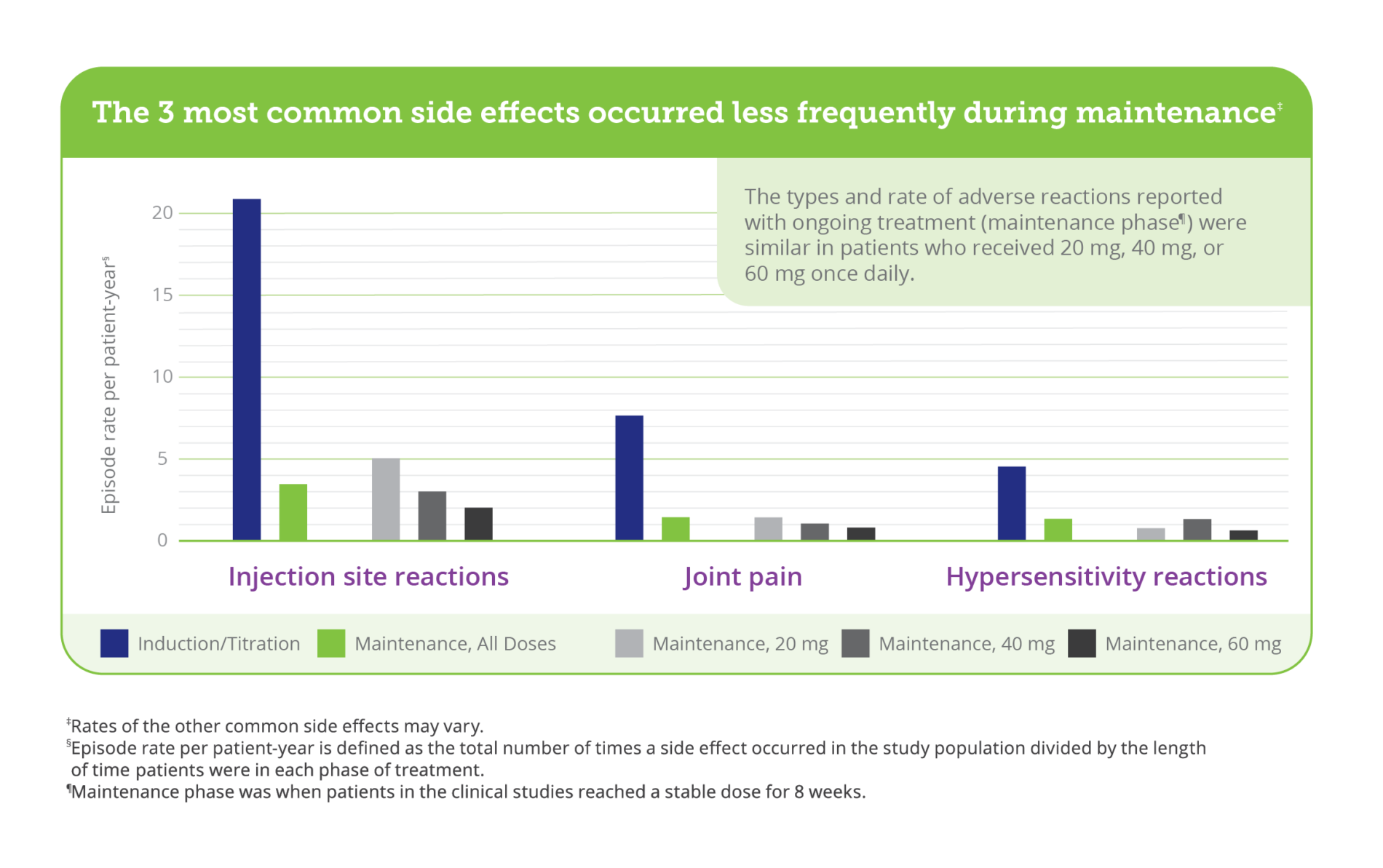
The most common side effects of PALYNZIQ include injection site reactions (such as redness, itching, pain, bruising, rash, swelling, or tenderness), joint pain, headache, skin reactions that spread and last at least 14 days (such as itching, rash, or redness), nausea, stomach pain, vomiting, cough, mouth and throat pain, itching, diarrhea, stuffy nose, feeling very tired, dizziness, anxiety, and low levels of Phe in your blood.
These are not all of the possible side effects of PALYNZIQ. Speak with your healthcare provider right away about any side effects.
Missed dose
Pregnancy Surveillance Program
You may report side effects to BioMarin at 1-866-906-6100.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please see full Prescribing Information, including an important warning for risk of anaphylaxis, and the Medication Guide.
COM-ET-0705 10/25
PALYNZIQ can cause a severe allergic reaction (anaphylaxis) that may be life threatening and can happen any time during treatment with PALYNZIQ.
What is PALYNZIQ?
PALYNZIQ® (Pal-lin-zeek) (pegvaliase-pqpz) is a prescription medication used to lower blood levels of phenylalanine (Phe) in adults with PKU (phenylketonuria) who have uncontrolled blood Phe levels above 600 micromol/L (10 mg/dL) on their current treatment. You should discuss the potential benefits and risks of PALYNZIQ with your healthcare provider.